In our previous blog post we have gone through all the steps to conduct a multi criteria decision analysis (MCDA). In this post we summarize its usefulness, the advantages and disadvantages, and we present two examples.
Why is conducting an MCDA useful?
- It is structured. It has a validated stepwise methodology. It always includes criteria, weights and scores.
- It is multidisciplinary. It incorporates the view of many experts.
- It is transparent. Everyone knows why you reached certain decision.
- It is systematic. You can do it over and over again.
- It incorporates several criteria. All relevant criteria are included in the decision process.
Of course, as any methodology, there are associated advantages and disadvantages. Here are a few examples.

Example 1: MCDA for a new drug in psoriasis
In a first example, a new drug for moderate to severe psoriasis has been evaluated. This was done before the price and funding were defined in Spain. The MCDA framework was adapted from EVIDEM. Six drug comparators were evaluated by a multidisciplinary committee of 12 experts, including patients, a nurse, and a psychologist [7]. Download the article here
The drug was assessed as an intervention with a positive value contribution in comparison to any of the alternative drugs. The most important value-added pieces were therapeutical benefits, health results reported by the patients, higher level of clearance, rapidity, and persistence with a similar safety and tolerability profile.
What was done in this MCDA which could not have been done with other tools?
- Comparison between 6 different alternatives in one exercise.
- Efficacy was broken down into three parts: clearance, rapidity, persistence. This is extremely specific to psoriasis.
- It is very rare that some types of professionals, such as nurses and psychologists, are included in the evaluation process, which has been done with this MCDA.
Here is a little taste of how results looked like.
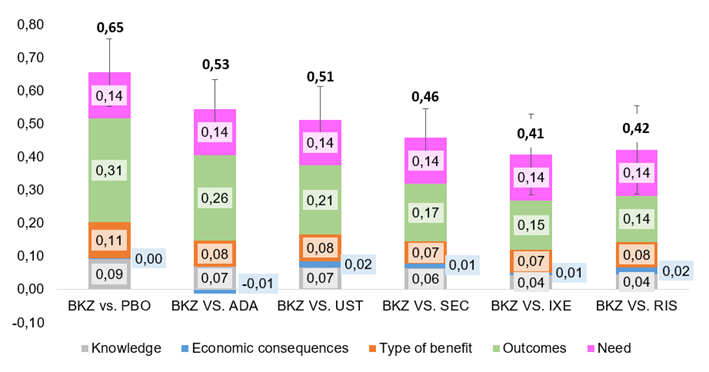
BKZ: Bimekizumab. PBO: placebo. ADA: Adalimumab. UST: Ustekinumab. SEC: Secukinumab. IXE: Ixekizumab. RIS: Risankizumab
Example 2: MCDA for procurement of vaccines
In order to find an ideal and sustainable framework for public procurement of vaccines in Spain, an MCDA was developed [5]. Download the article here.
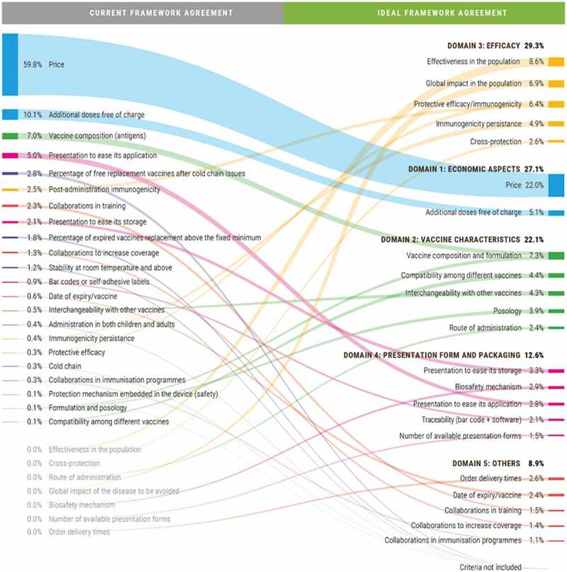
In summary, steps 1 through 6 were carried out (see previous blog post). A framework was developed, and an expert committee defined the weight each criterion should have. They compared the current scenario with the ideal scenario, in terms of criteria and weight.
While the price had a weight of almost 60% in the current scenario, the ideal framework defined it should have a weight of only 22%. Basically, what this MCDA allowed was, amongst other things, to show that experts believe that the value is not limited to pricing, but relies on many other criteria as well.
Take a look…
Now, you try it
Go forth and try using an MCDA as a complement to other tools.
Let us know how it worked, and if they have helped you in making complex decisions.
